Is the Hydrogen Wave Function Real or Just Math?
Introduction
The hydrogen wave function is one of the most significant results of quantum mechanics. It provides a mathematical description of the electron's behavior in a hydrogen atom, offering profound insights into atomic structure and interactions. Yet, fundamental questions linger: Is the wave function a real, physical entity, or merely a mathematical abstraction to predict experimental results? This article explores the historical development, acceptance, criticisms, and limitations of the hydrogen wave function, ultimately highlighting its dual nature as both a mathematical construct and a powerful descriptor of reality.
Historical Development
Early Atomic Models
Before the advent of quantum mechanics, atomic models were simplistic and unable to explain certain experimental results. For instance:
- John Dalton's Atomic Theory (1803): Proposed that atoms are indivisible particles, but lacked any mechanism to describe their internal structure.
- J.J. Thomson's Plum Pudding Model (1904): Depicted atoms as spheres of positive charge embedded with electrons, which failed to explain spectral lines.
Bohr Model and Its Shortcomings
In 1913, Niels Bohr introduced his model of the hydrogen atom. Bohr's model proposed quantized orbits for electrons, successfully explaining the spectral lines of hydrogen. However, it could not account for:
- Fine structure in spectral lines.
- The behavior of atoms with more than one electron.
Schrödinger’s Wave Mechanics
In 1926, Erwin Schrödinger formulated his wave equation, revolutionizing atomic theory. The hydrogen wave function, ψ(r, θ, ϕ), emerged as a solution to this equation. Schrödinger’s work bridged the gap between classical mechanics and quantum phenomena, showing that the electron in a hydrogen atom could be described as a wave-like entity rather than a particle confined to specific orbits.
Experimental Validation
The wave function’s predictions were validated by:
- Hydrogen Spectra: The calculated energy levels matched the observed spectral lines with unprecedented accuracy.
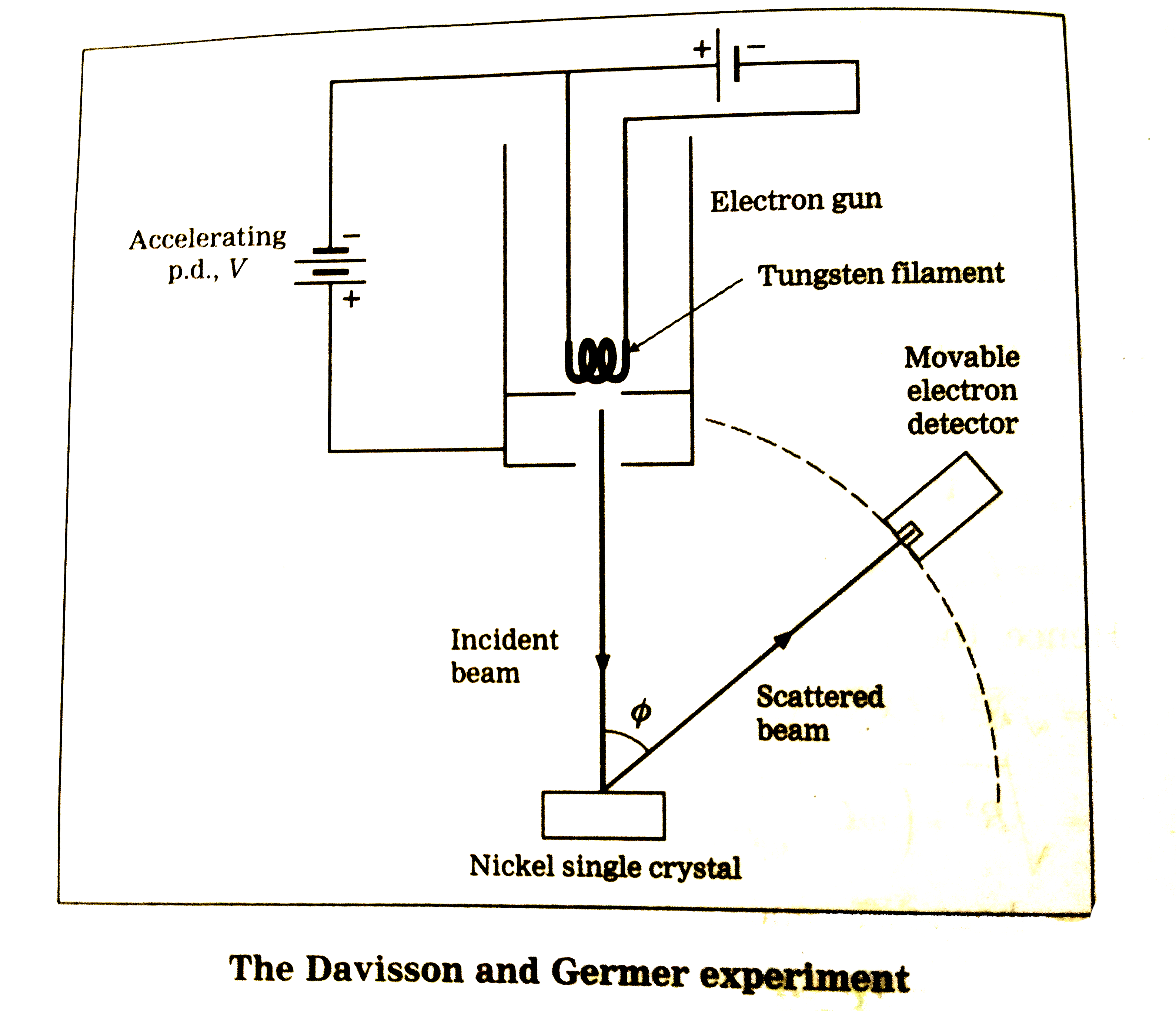
- Electron Diffraction: Experiments by Davisson and Germer (1927) confirmed the wave-like behavior of electrons by observing diffraction patterns when electrons were scattered off a nickel crystal. This experiment demonstrated that electrons, traditionally considered particles, exhibit wave-like properties consistent with Schrödinger's predictions. Below is an image illustrating their experimental setup and results:
The diffraction pattern provided direct evidence of the wave nature of electrons, lending strong experimental support to the quantum mechanical framework.
Why Was the Hydrogen Wave Function Accepted?
-
Mathematical Consistency
- The hydrogen wave function solved the Schrödinger equation, which itself was based on fundamental principles of quantum mechanics.
- Its solutions provided quantized energy levels, directly corresponding to experimentally observed spectral lines.
-
Predictive Power
- The wave function predicted phenomena like orbital shapes (e.g., spherical -orbitals, dumbbell-shaped -orbitals) that matched experimental evidence.
-
Technological Applications
- The wave function underpins technologies such as lasers, semiconductors, and quantum computing, demonstrating its practical relevance.
Criticisms and Limitations
Despite its success, the hydrogen wave function is not without criticism.
Philosophical Criticisms
-
Copenhagen Interpretation
- According to this interpretation, the wave function is merely a tool for calculating probabilities, not a real physical entity.
- Critics argue that the wave function does not describe what the electron “is”, only where it might be found.
-
Einstein’s Objections
- Albert Einstein famously remarked, “God does not play dice,” expressing skepticism about the probabilistic nature of the wave function.
-
Realist Interpretations
- Realists like proponents of the Pilot Wave Theory suggest that the wave function is incomplete, requiring additional hidden variables to describe reality fully.
Problems the Wave Function Cannot Describe
-
Multi-electron Systems
- For atoms beyond hydrogen, solving the Schrödinger equation becomes computationally infeasible due to electron-electron interactions.
-
Quantum Gravity
- The wave function fails to reconcile with general relativity, highlighting the need for a unified theory.
-
Measurement Problem
- The wave function does not explain how or why it collapses during a measurement, leading to unresolved debates.
Intriguing Questions
- What does the wave function collapse represent? Is it a physical process or an artifact of observation?
- Can the wave function describe systems on macroscopic scales?
- If the wave function is real, where does it exist? In physical space, configuration space, or some abstract mathematical realm?
Is the Wave Function Real or Just Math?
The Argument for Reality
- The wave function’s predictions match experimental results with incredible precision.
- Phenomena like quantum tunneling and orbital shapes are directly derived from the wave function and observed experimentally.
The Argument for Math
- The wave function is a complex-valued function, often requiring imaginary numbers, which have no direct physical interpretation.
- Its probabilistic nature suggests it is a tool for prediction, not a description of reality itself.
Conclusion
The hydrogen wave function occupies a unique place in science: it is a mathematical model that describes reality with extraordinary precision. Yet, like all models, it is an abstraction and may not fully represent the underlying nature of reality. The wave function’s success lies in its ability to predict experimental outcomes, even if its true nature remains elusive.
Ultimately, whether the hydrogen wave function is “real” or just math may not matter for practical purposes. What matters is that it works. However, as we probe deeper into the quantum world, new discoveries may reveal its limitations or even point to a more comprehensive theory that supersedes it.